Which of the Following Elements Has the Smallest Atomic Radius
Which of the following elements has the smallest atomic radius. What ions has smallest radius.
Hii I think that it helps you.

. Asked Sep 3 2019 in Chemistry by Emagee. Rank the atomic radius from largest to smallest. - Iodine or I coes on.
Which element has the smallest atomic radius. Fluorine F has the smallest atomic radius among the given elements. 2 He - Helium.
Chlorine has the smallest atomic radius and the highest ionization energy. Which of the following elements has the smallest atomic radius. 3 Li - Lithium.
To rank items as equivalent overlap them. And thus the element with the smallest atomic radius should be helium Z2. Atomic radii change in a predictable way throughout the periodic table.
Down the group size increases due to addition of a new shell. Atomic radius decreases tired so from left to right in decreasing and from down the group write down the group it increases this is our atomic radius so the question is which one of the following is smallest atomic radius rate so if we observed that from Elite wap it decreases so it decreases f is the smallest what is the look about chlorine chlorine is down to the atomic radius of chlorine. What element in the second period has the largest atomic radius.
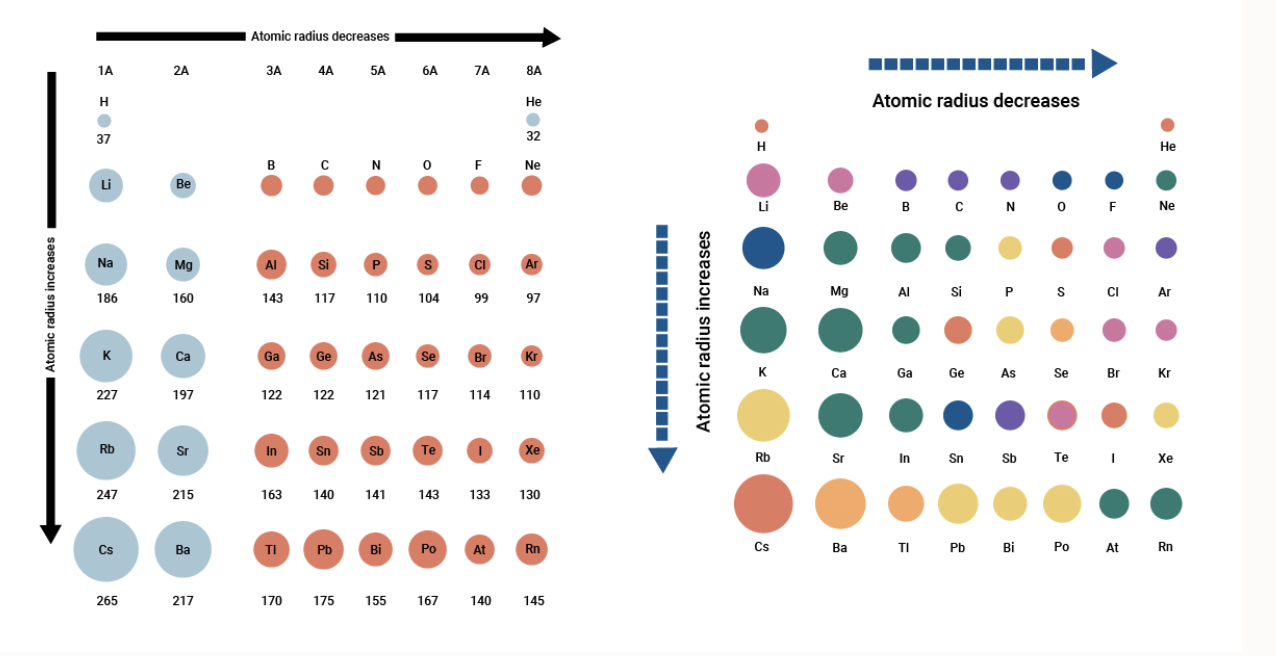
Therefore helium is the smallest element and francium is the largest. Atomic radius of Hydrogen H. As shown in the figure below the atomic radius increases from top to bottom within the group and decreases from left to right over the entire period.
Which of the following statements is true about. See the answer See the answer See the answer done loading. The atomic size decreases towards the right in a period as effective nuclear charge increases.
Element Atomic Number Element Symbol Element Name Element Element Atomic Radius 1. Atomic radii represent the sizes of isolated electrically-neutral atoms unaffected by bonding topologies. As you move across a single period row on the periodic table the.
Sulfur Chlorine Selenium Bromine See periodic table. Thus helium is the smallest element and francium is the largest. Calcium potassium scandium titanium Which of the transition metals has the smallest atomic radius The atomic mass of titanium is 4788 atomic mass units.
Atomic radii decrease however as one moves from left to right across the Periodic Table. This atomic mass represents the. Which of the following elements has the smallest atomic radius bromine selenium chlorine sulfur.
Asked May 8 2020 in Chemistry by Cazetta. Which element has the smallest atomic radius in order. This problem has been solved.
This site reports that the atomic radius is 31 1012m where the atomic radius of the hydrogen atom is 53 1012m. Because K has the greatest nuclear charge Z 19 its radius is smallest and S 2 with Z 16 has the largest radius. Place the following elements in order of decreasing atomic radius.
Since The element which will in the right most of the Theriodic table will be having smallest alomic radius. Thus the order of size is NaMgBN. Up to date curated data provided by Mathematica s ElementData function from Wolfram Research Inc.
Hence the smallest is NNitrogen. Which of the following elements has the smallest atomic radius. Chlorine Cl lead Pb aluminum Al and fluorine F.
What is the charge of a cation. Helium Atomic radii vary in a predictable way across the periodic table. The right most comer of perioder and it belongs to Group 17 and we know that across the period aromir rodres decreases hence correct option is i.
119 rows Atomic number Elements Atomic Radius of Elements pm 1. 1 H - Hydrogen. Which of the following elements has the smallest atomic radius.
The general trend is that atomic sizes increase as one moves downwards in the Periodic Table of the Elements as electrons fill outer electron shells. As can be seen in the figures below the atomic radius increases from top to bottom in a group and decreases from left to right across a period. Which of the following atoms has the smallest atomic radius.
Chlorine selenium and bromine are located near each other on the periodic table. Thus helium is the smallest element and francium is the largest. That atomic size should decrease across a Period from left to right as we face the Table can be attributed to the increase of nuclear charge ie.
As can be seen in the figures below the atomic radius increases from top to bottom in a group and decreases from left to right across a period. Which of the following elements has the smallest atomic radius. Which of these elements is the smallest atom and which has the highest ionization energy.

Free Cheat Sheet For Chemistry Lovers Chemistry Education Teaching Middle School Science Chemistry Lessons

Solved Answer Each Of The Following Questions A Which Of The Elements P A S And S Has The Largest Atomic Radius A Which Of The Elements P A S And S Has

Among The Elements Ca Mg P And Cl The Order Of Increasing Atomic Radii Is

The Periodic Table Of The Elements Trends In Atomic Radius Electronegativity Ionizati Ionization Energy Chemistry Worksheets Periodic Table Of The Elements
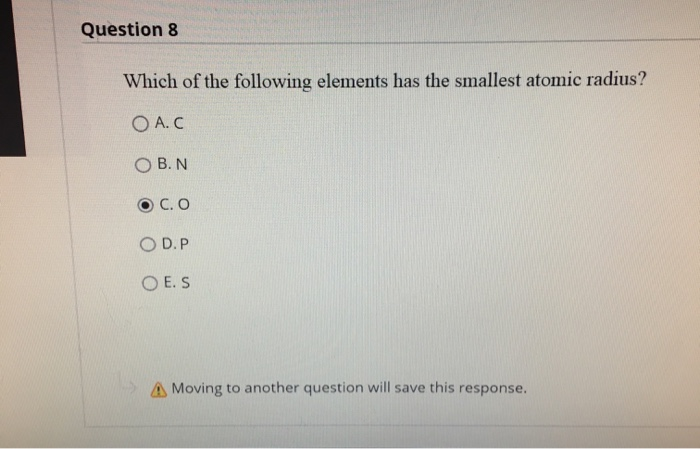
Solved Question 8 Which Of The Following Elements Has The Chegg Com
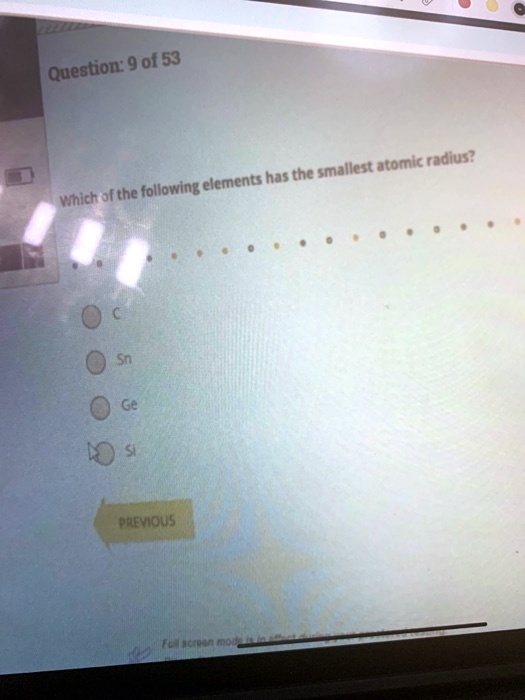
Solved Question 9 0f 53 Smallest Atomic Radius Elements Has The Which Of The Following Paeviqus

Solved Which Of The Following Elements Has The Smallest Chegg Com

Question Video Identifying Which Element Has An Atomic Radius Larger Than Aluminum Nagwa
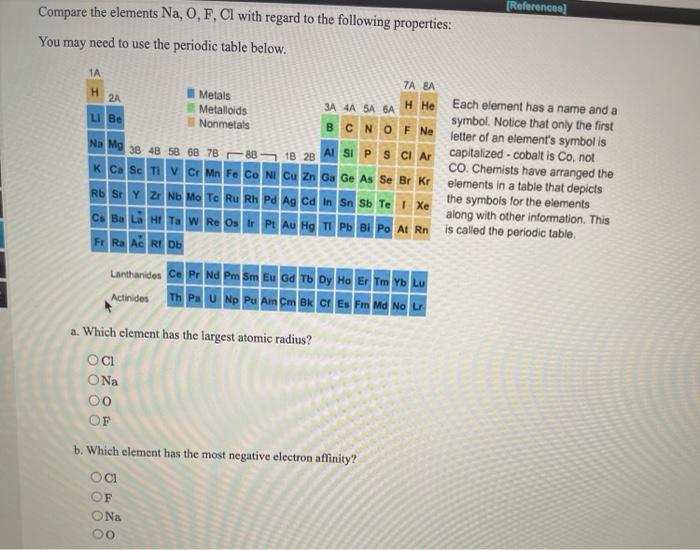
Solved A In The Following Set Which Atom Has The Smallest Chegg Com

High School Chemistry Atomic Size Wikibooks Open Books For An Open World

Discovering Atomic Radius Ws Effective Nuclear Charge Atom Discover
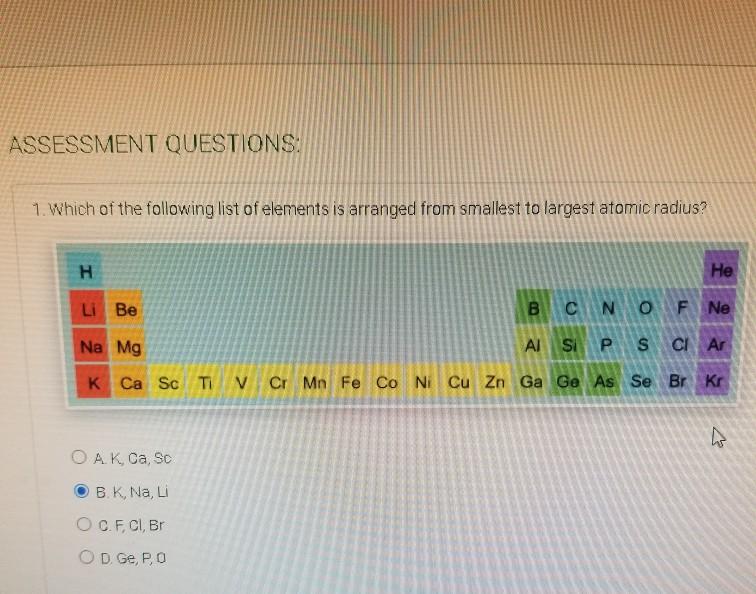
Solved Assessment Questions 1 Which Of The Following List Chegg Com

Periodic Trends Determine Which Atom Has The Smallest Atomic Radii Radius Johnny Cantrell Youtube

Pin By Pieter De Kooker On Chemistry Chemistry Electrons Study
Solved 15 Which Of These Elements Has The Smallest Atomic Chegg Com

What Is Atomic Radius In Chemistry Tutordale Com

Periodic Trends Review Problems Mass Atomic Radius Electronegativity Etc In 2022 Ionization Energy Electron Configuration Atom


Comments
Post a Comment